In adults with Type 1 diabetes (T1D), as an adjunct to insulin,
THE DEPICT PROGRAMME IS THE FIRST* PHASE III TRIAL TO BE REPORTED USING A SELECTIVE SGLT-2i1
*In the EU.
swipe to view →
Forxiga 10 mg is not licensed in the EU for the treatment of T1D.
**Forxiga 5 mg is only indicated in adult T1D patients with a BMI of ≥27 kg/m2.4
BMI, body mass index; C-peptide, connecting peptide; CGM, continuous glucose monitor; CrCl,
creatinine clearance; HbA1c, haemoglobin A1c; IU, international units; T1D, Type 1 diabetes.
In adults with T1D, as an adjunct to insulin
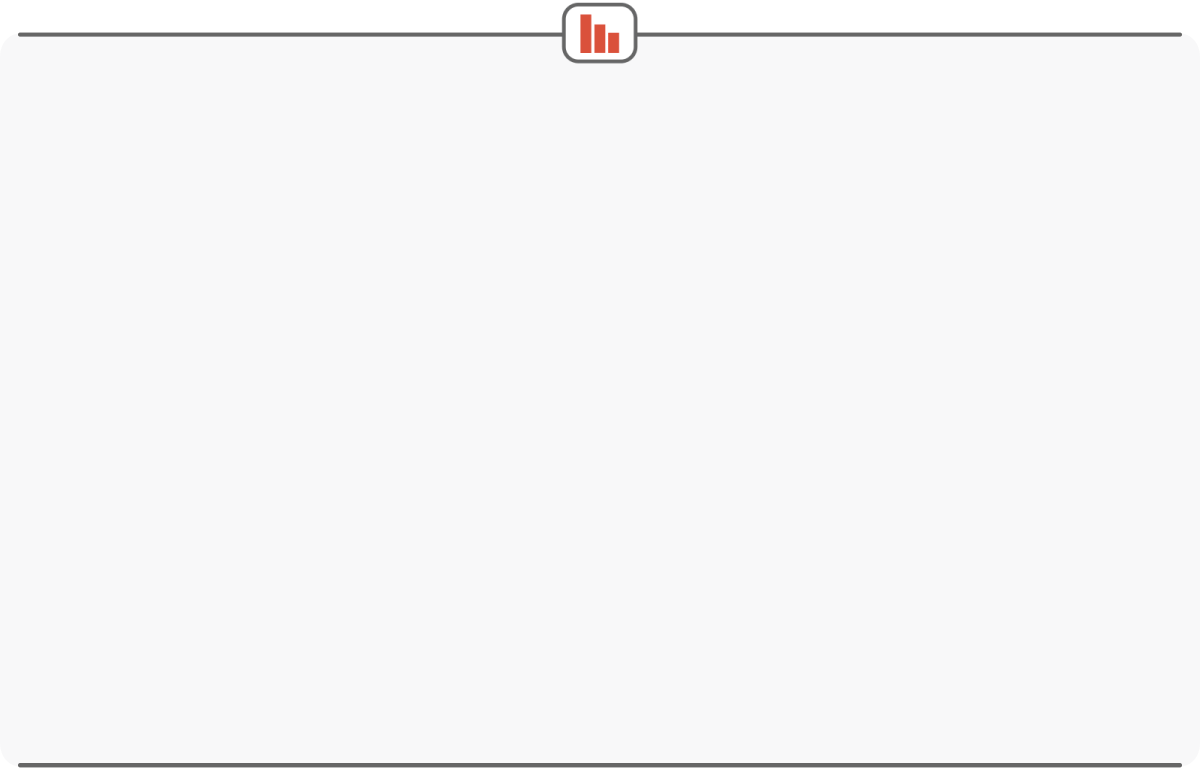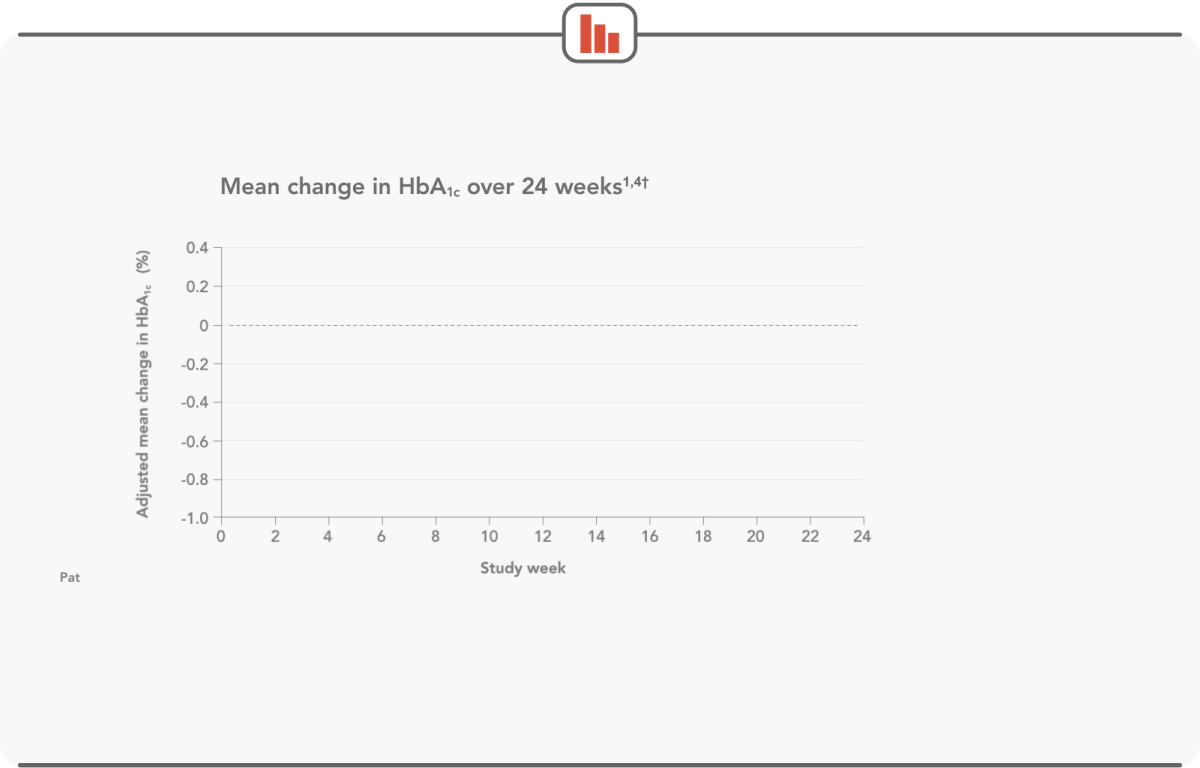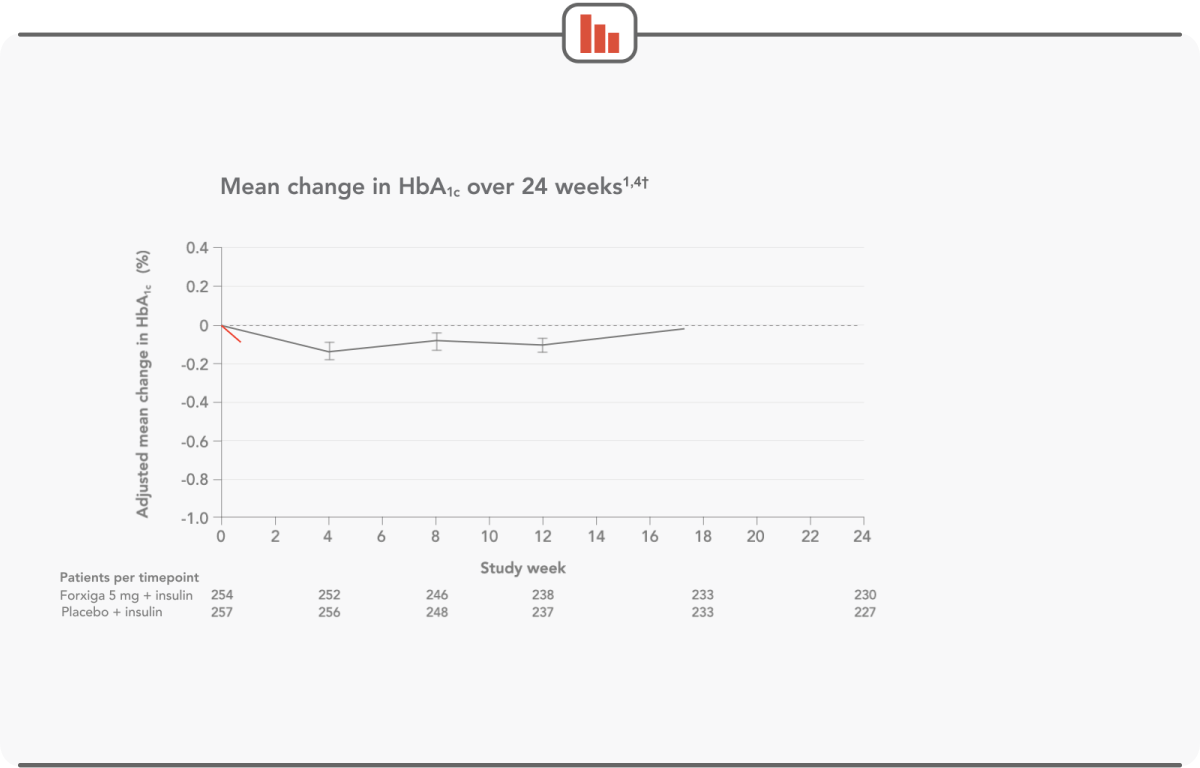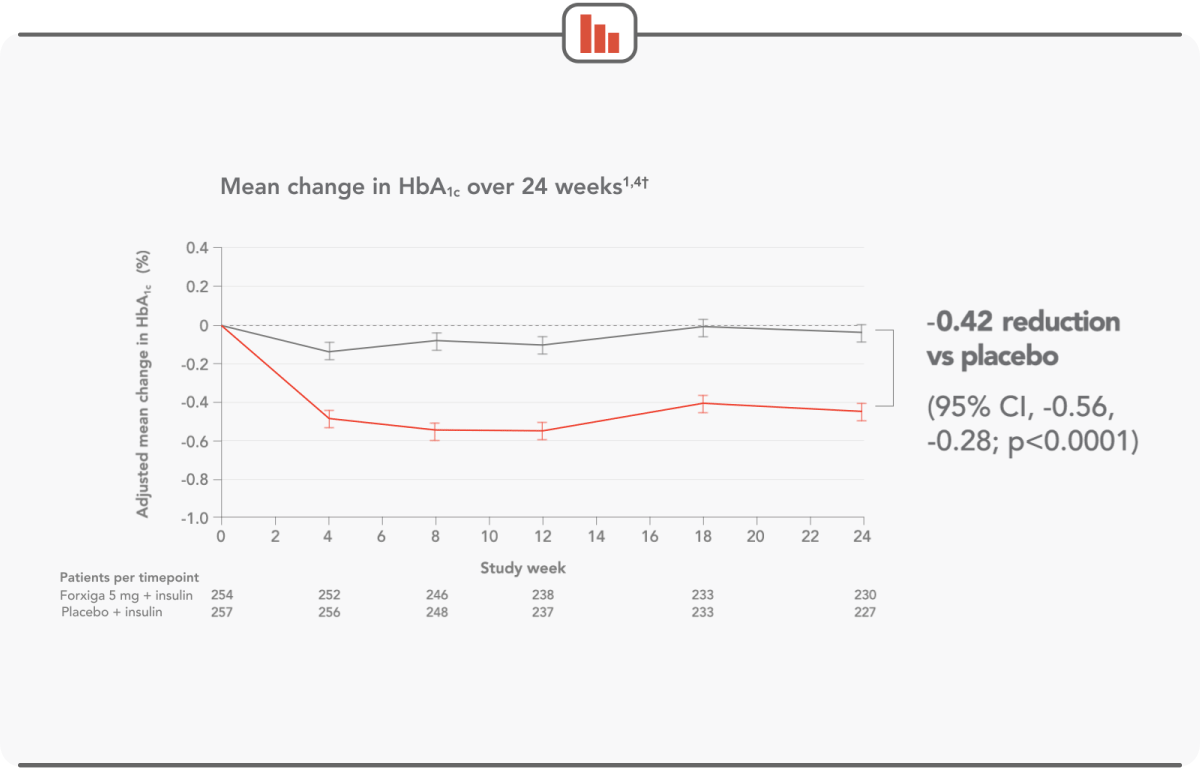
FORXIGA 5 mg DELIVERED CLINICALLY RELEVANT HbA1c REDUCTIONS
swipe to view →
Forxiga 5 mg + insulin: (n=259) mean baseline HbA1c was 8.52%; mean baseline total daily insulin dose was 62.91 IU. Placebo + insulin: (n=260) mean baseline HbA1c was 8.50%; mean baseline total daily insulin dose was 61.74 IU.
†The information presented here is from DEPICT-1. Efficacy was also demonstrated in DEPICT-2, which showed HbA1c reductions of -0.37% (P<0.0001).4 CI, confidence interval; HbA1c, haemoglobin A1c; IU, international units.
Patients saw significant HbA1c reductions as early as week 4, with results sustained throughout the 24-week trial1,4
In adults with T1D, as an adjunct to insulin
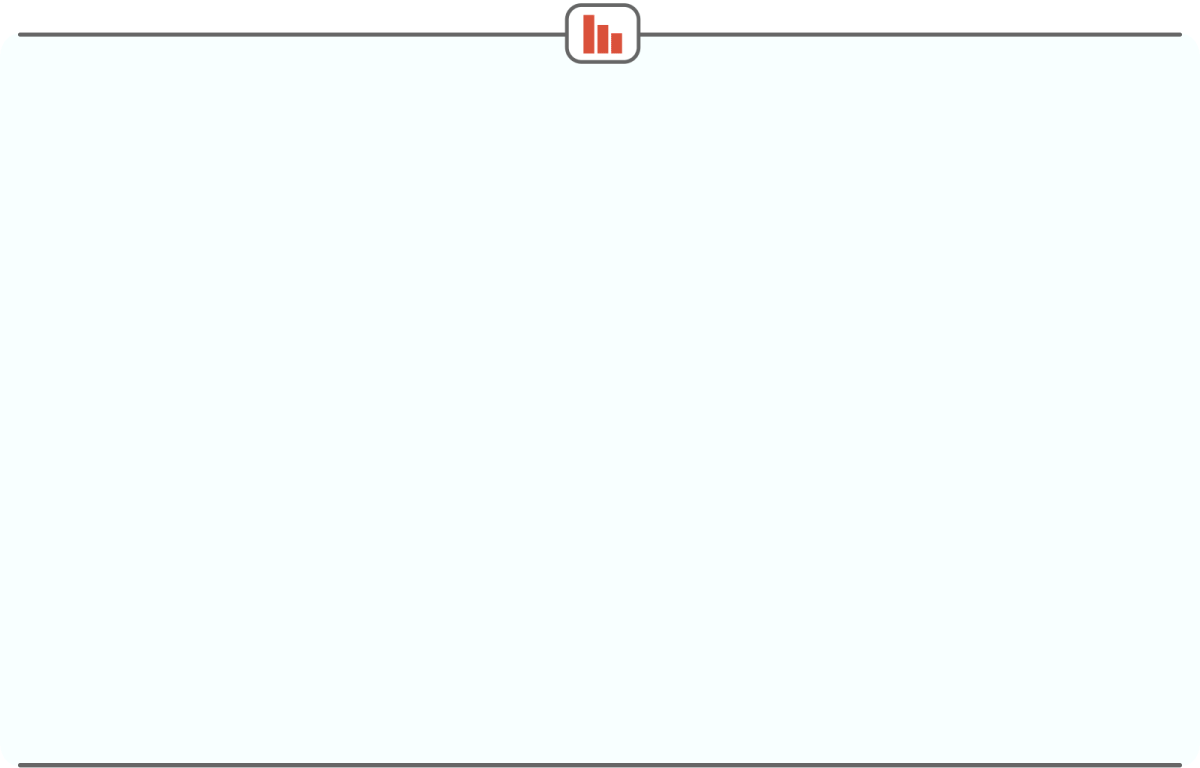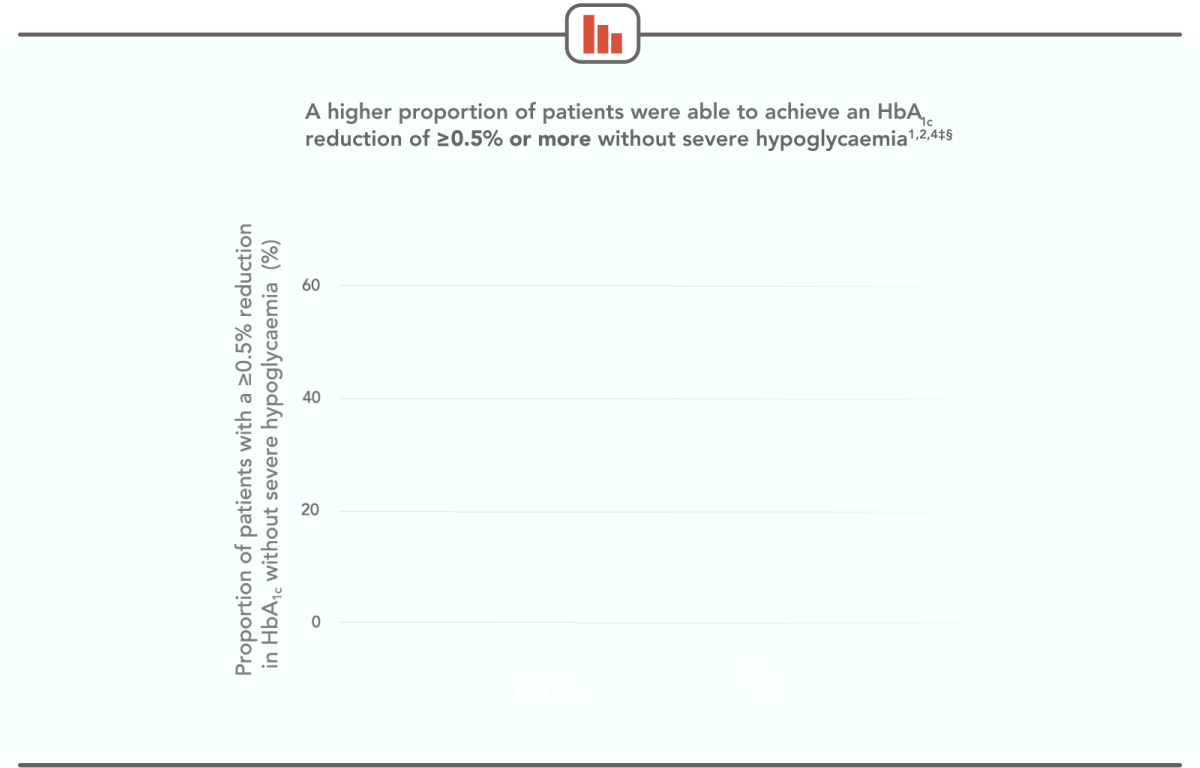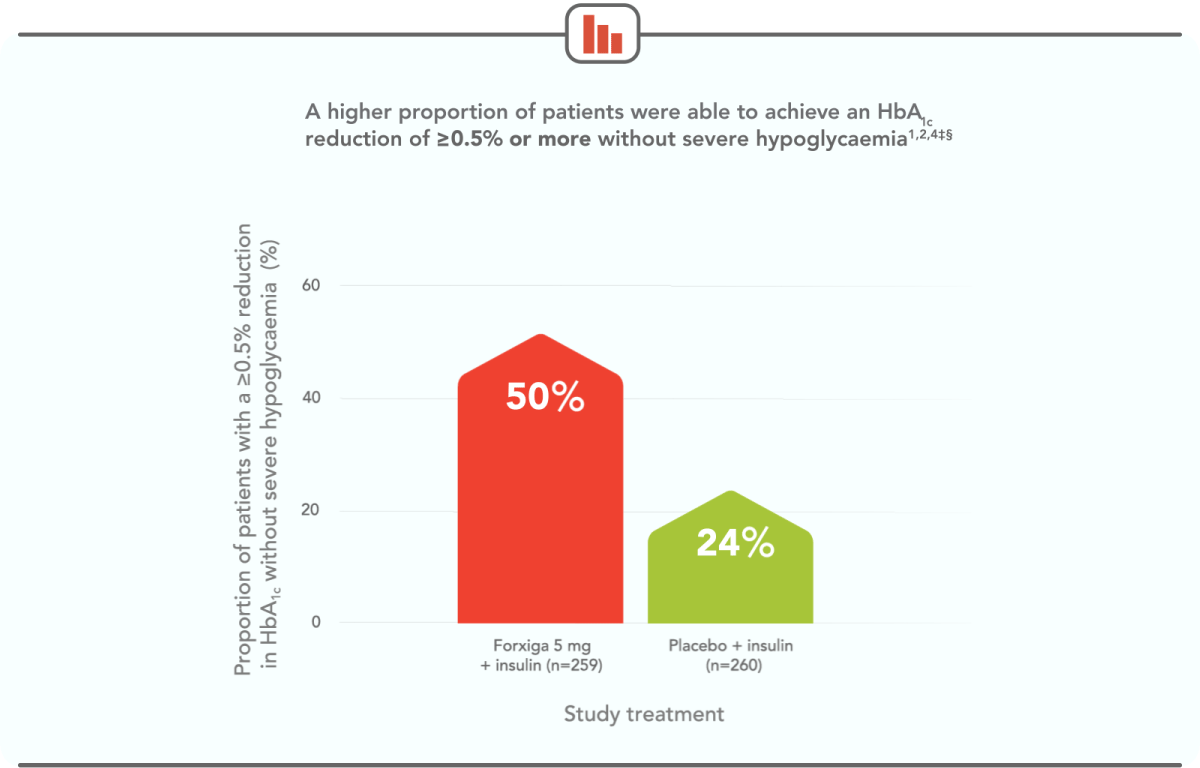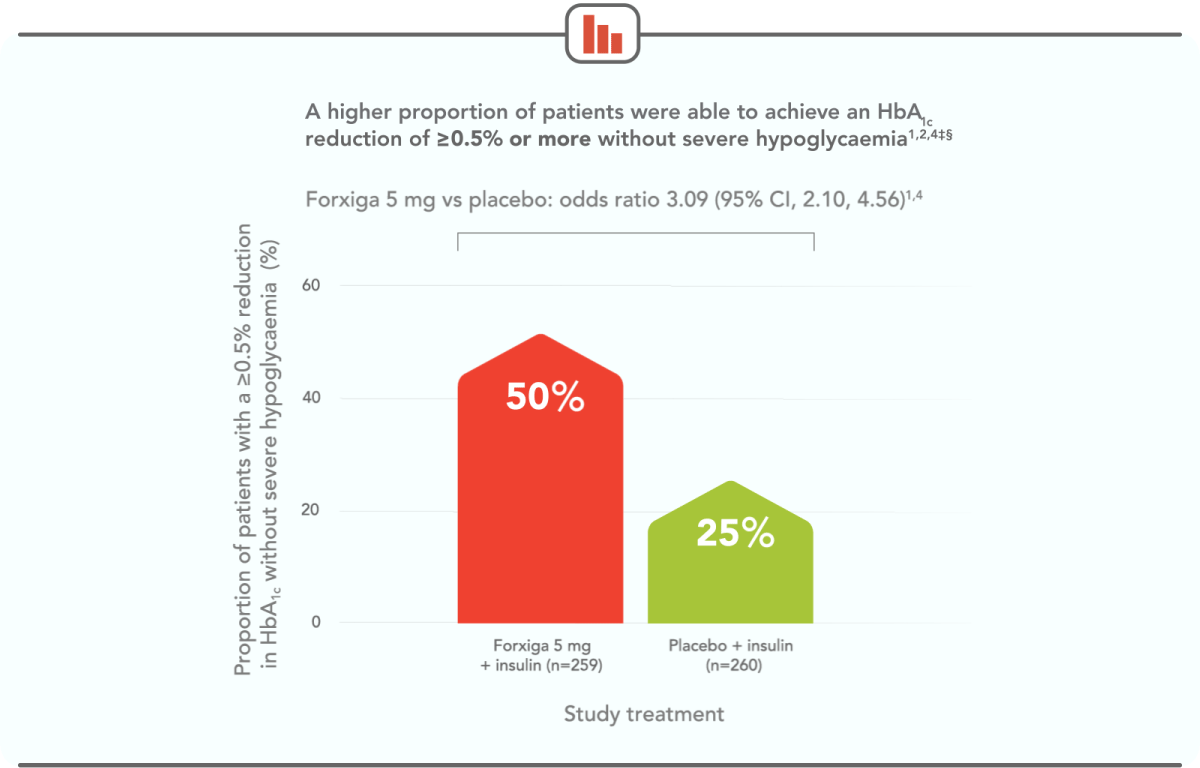
TWICE AS MANY PATIENTS ACHIEVED ≥0.5% HbA1c REDUCTION WITHOUT SEVERE HYPOGLYCAEMIA WITH FORXIGA 5 mg VS PLACEBO AT 24 WEEKS1,4‡§
‡The information presented here is from DEPICT-1. DEPICT-2 also demonstrated a higher proportion of Forxiga patients achieving this outcome: 39.5% in the Forxiga 5 mg arm and 20.1% in the placebo arm (odds ratio 2.71; 95% Cl, 1.81, 4.06).1,3
§Severe hypoglycaemia required assistance of another person to raise glucose levels and promote neurological recovery.5
CI, confidence intervals; HbA1c, haemoglobin A1c
In adults with T1D, as an adjunct to insulin
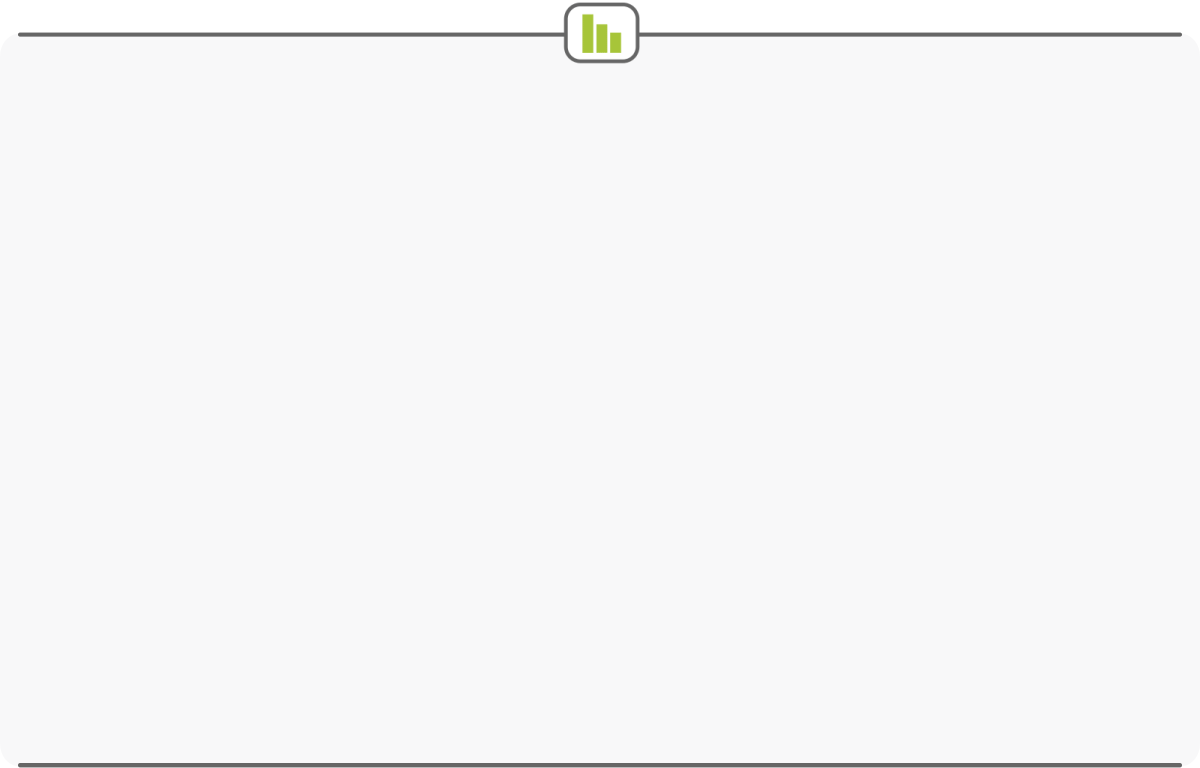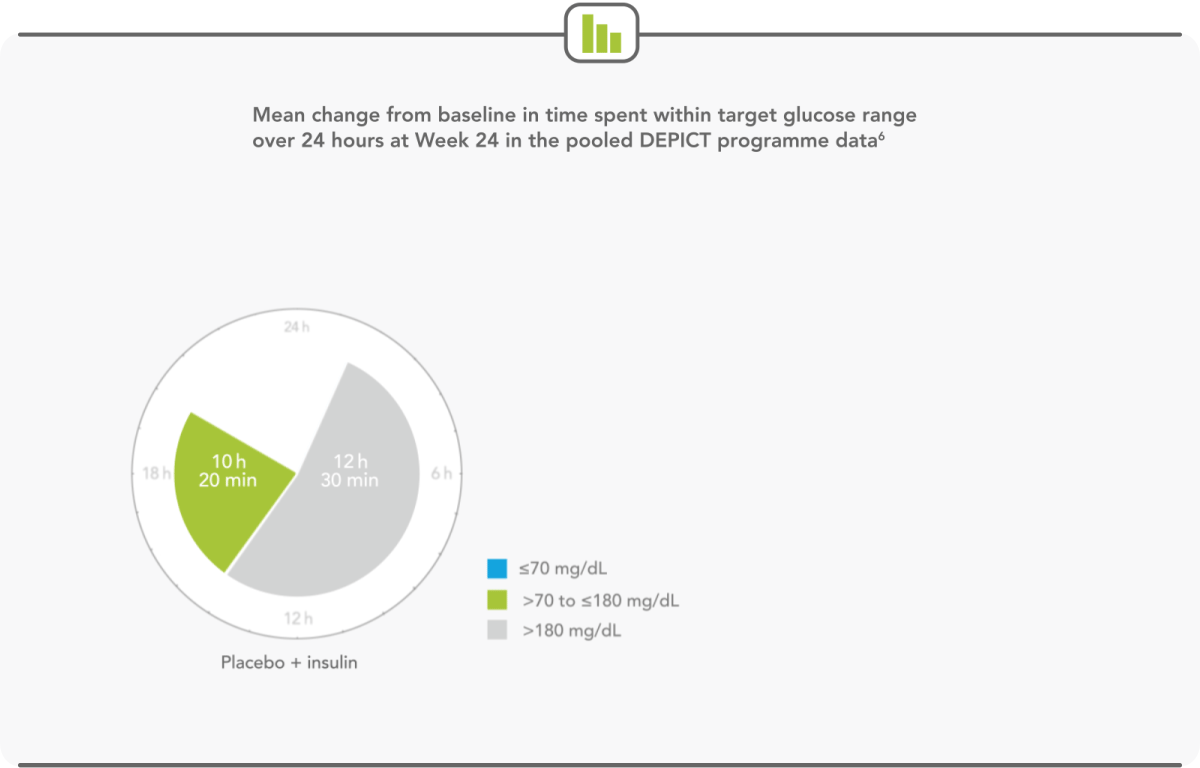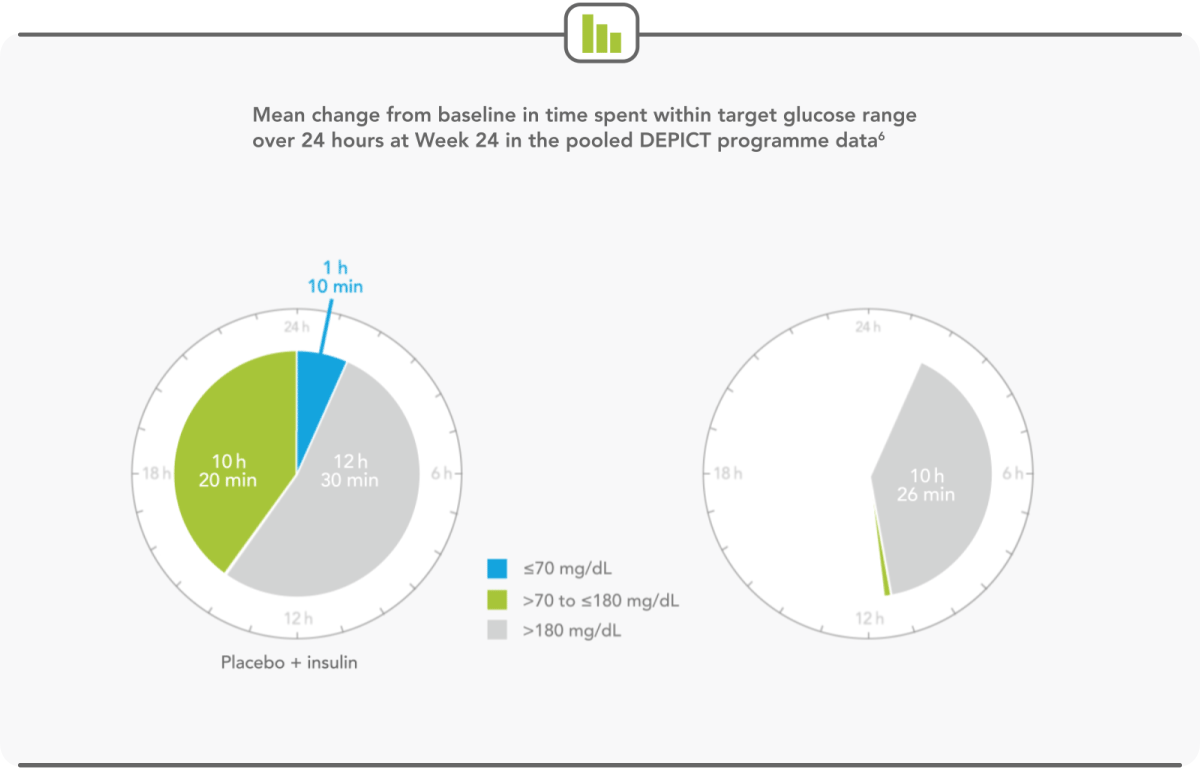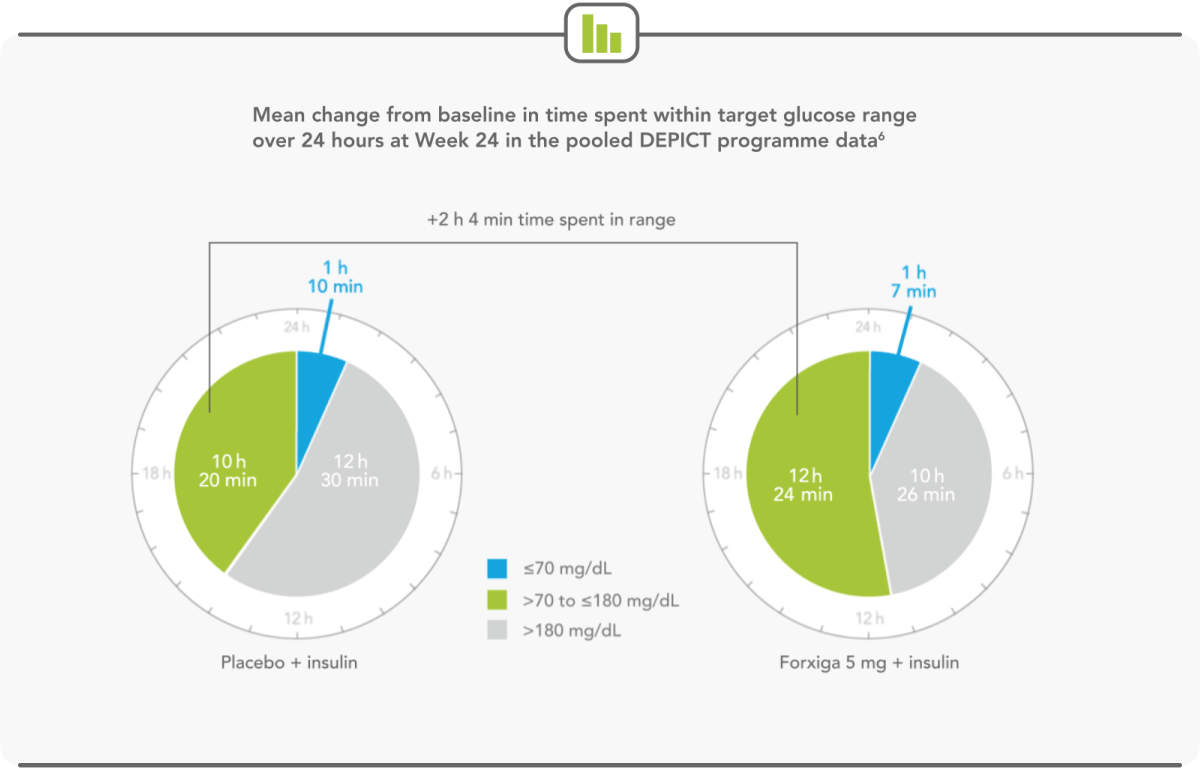
PATIENTS SPEND MORE THAN HALF THEIR DAY WITHIN TARGET GLUCOSE RANGE WITH FORXIGA 5 mg6
- Forxiga 5 mg extended the time a patient spent in target glycaemic range by more than 2 hours per day6
- Forxiga 5 mg led to relative increases in mean continuous glucose-monitoring values in the target glucose range of 20%6
In adults with T1D, as an adjunct to insulin,
FORXIGA 5 mg OFFERS PATIENTS THE ADDITIONAL BENEFIT OF WEIGHT REDUCTIONS1,4¥
swipe to view →
Forxiga 5 mg + insulin (n=259): (n=259) mean baseline body weight 81.67 kg4 Placebo + insulin (n=260): (n=260) mean baseline body weight was 84.42 kg4
¥Forxiga is not indicated for the management of weight loss. Weight change was a secondary endpoint in clinical trials.
- The information presented here is from DEPICT-1. DEPICT-2 also showed reductions of 2.5 kg with Forxiga 5 mg (p<0.0001 vs placebo)1,3
- Body weight reductions mostly occurred within the first 8 weeks and continued thereafter, reaching almost 3 kg by Week 24 (P<0.0001)1
References:
- Dandona P, Mathieu C, Phillip M, et al; on behalf of the DEPICT-1 Investigators. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (DEPICT-1): 24 week results from a multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5:864-876.
- Mathieu C, Dandona P, Gillard P, et al. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (the DEPICT-2 Study): 24-week results from a randomized controlled trial. Diabetes Care. 2018 (suppl):10.2337/dc18-0623.
- Mathieu C, Dandona P, Gillard P, et al. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (the DEPICT-2 Study): 24-week results from a randomized controlled trial. Diabetes Care. 2018;41:1938-1946.
- Forxiga. Summary of Product Characteristics. 2019. AstraZeneca.
- Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40(12):1631–1640.
- Rudofsky G. Long-term efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes—the DEPICT-2 study. Presented at: 18th International Congress of Endocrinology; December 1-4, 2018; Cape Town, South Africa.